Abstract
Background: Patients with multiple myeloma (MM) that are refractory to a proteasome inhibitors (PI), immunomodulatory drug (IMiDs) and CD38/SLAMF7 directed monoclonal antibodies are commonly referred to as triple class refractory (TCR). This is an increasingly common clinical problem and here we present the outcomes, prognostic markers and response to therapy for patients with TCR disease.
Methods: Consecutive patients with MM diagnosed between 01/01/2013 and 12/31/2018 and seen at Mayo Clinic, Rochester, MN were included in this study after institutional review board approval. Refractoriness to therapy was defined as progression or non-responsiveness (failure to achieve at least a minimal response) to therapy within 60 days of discontinuation of therapy, based on the International Myeloma Workshop Consensus definition. Patients meeting the definition for refractory disease for at least 1 PI (bortezomib, carfilzomib or ixazomib), 1 IMiD (lenalidomide, pomalidomide or thalidomide) and 1 monoclonal antibody (daratumumab, isatuximab or elotuzumab) were considered to have TCR disease and included in the study population. The first date when patients satisfied the above-mentioned criteria for TCR was used to calculate the follow-up, progression free survival (PFS) and OS for TCR disease. The mSMART 3.0 (www.msmart.org) classification was used to characterize high-risk and standard-risk cytogenetics features in interphase fluorescence in situ hybridization (FISH). The currently prevalent international myeloma working group (IMWG) criteria were used for response assessment. The Kaplan-Meier method was used for estimation of time-to-event endpoints. Reverse censoring was used for estimation of follow-up time. Cox regression was used for multivariate analyses. Wilcoxon test was used for comparison of continuous variables and Chi square test or Fisher's exact test were used to compare categorical variables, respectively
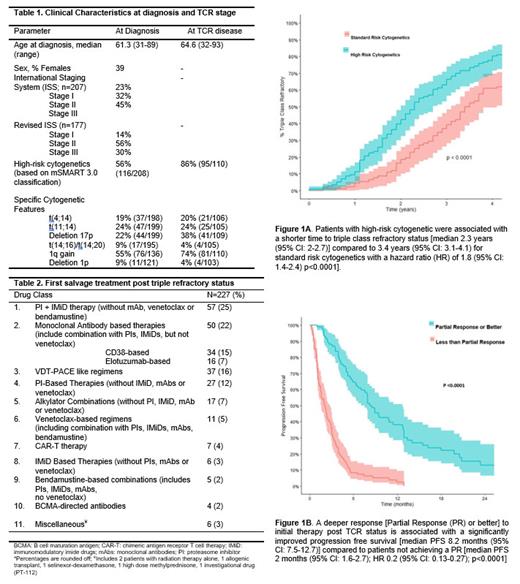
Results: In this retrospective study, 249 consecutive patients with TCR status were identified. The median follow-up from diagnosis of MM till date of last follow-up was 6.8 years (95% CI: 6.3-7.3 years) and 1.9 years (95% CI: 1.6-2.4) from TCR MM to last follow-up. The median age at diagnosis of MM was 61 years (range 31-89) and at TCR was 65 years (range 32-93). The median number of lines of therapy for the entire cohort were 8 (range 1-17); the patients were exposed to a median of 5 (range 1-12) lines of therapy prior to TCR status. Baseline characteristics for the cohort are depicted in Table 1. The median time to development of triple refractory disease was 2.9 years (95% CI: 2.6-3.2 years) from diagnosis of MM. The time to TCR disease was shorter for high-risk cytogenetics at 2.3 years (95% CI: 2-2.7) compared to 3.4 years (95% CI: 3.1-4.1) with a hazard ratio (HR) of 1.8 (95% CI: 1.4-2.4) p<0.0001], Figure 1A. The median overall survival (OS) from TCR was 1.01 (95% CI: 0.9-1.3) years. High-risk cytogenetics at diagnosis of MM were associated with an inferior OS from TCR disease even after adjustment for age, and ISS stage [median 1.05 years (95% CI: 0.8-1.5) for high-risk vs 1.3 years (95% CI: 0.9-2.9) for standard-risk cytogenetics; hazard ratio 1.44 (95% CI: 1.01-2.05); p=0.04]. Of 249 patients, 227 had data available for therapy post TCR (initial therapy details post TCR status are depicted in Table 2) with a median PFS of 4 months (95% CI: 3.5-5) for the salvage therapy post attainment of TCR. Patients achieving a deeper response [≥partial response (PR)] were noted to have a significantly improved PFS with the initial therapy post TCR disease [median PFS 8.2 months (95% CI: 7.5-12.7)] compared to patients not achieving a PR [median PFS 2 months (95% CI: 1.6-2.7); HR 0.2 (95% CI: 0.13-0.27); p<0.0001], Figure 1B. Deeper response (≥PR) to initial therapy at TCR disease was associated with an improved PFS [HR 0.23 (95% CI: 0.16-0.34); p <0.0001] after adjusting for age at TCR status, ISS stage and cytogenetic risk categories at diagnosis.
Conclusion: Patients with triple refractory MM have a guarded prognosis and demonstrate an underwhelming median PFS of 4 months and median OS of 1 year. Achievement of a deeper response to therapy at triple refractory status is associated with a prolonged PFS. High-risk cytogenetic features at diagnosis confer a negative prognosis in TCR patients. Newer therapies are the needed to sustain the improvement in outcomes for this group of patients.
Kapoor: Cellectar: Consultancy; Pharmacyclics: Consultancy; Sanofi: Consultancy; BeiGene: Consultancy; Amgen: Research Funding; Ichnos Sciences: Research Funding; Karyopharm: Consultancy; Regeneron Pharmaceuticals: Research Funding; AbbVie: Research Funding; Takeda: Research Funding; Sanofi: Research Funding; Karyopharm: Research Funding; Glaxo SmithKline: Research Funding. Dispenzieri: Pfizer: Research Funding; Sorrento Therapeutics: Consultancy; Oncopeptides: Consultancy; Alnylam: Research Funding; Takeda: Research Funding; Janssen: Consultancy, Research Funding. Gertz: Ionis Pharmaceuticals: Other: Advisory Board; Akcea Therapeutics, Alnylam Pharmaceuticals Inc, Prothena: Consultancy; AbbVie Inc, Celgene Corporation: Other: Data Safetly & Monitoring; Aurora Biopharma: Other: Stock option; Akcea Therapeutics, Ambry Genetics, Amgen Inc, Celgene Corporation, Janssen Biotech Inc, Karyopharm Therapeutics, Pfizer Inc (to Institution), Sanofi Genzyme: Honoraria. Dingli: Apellis: Consultancy; GSK: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; Novartis: Research Funding; Alexion: Consultancy. Kumar: Sanofi: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astra-Zeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Consultancy; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche-Genentech: Consultancy, Research Funding; Beigene: Consultancy; Tenebio: Research Funding; Novartis: Research Funding; Adaptive: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Merck: Research Funding; Carsgen: Research Funding; Antengene: Consultancy, Honoraria; Oncopeptides: Consultancy; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal